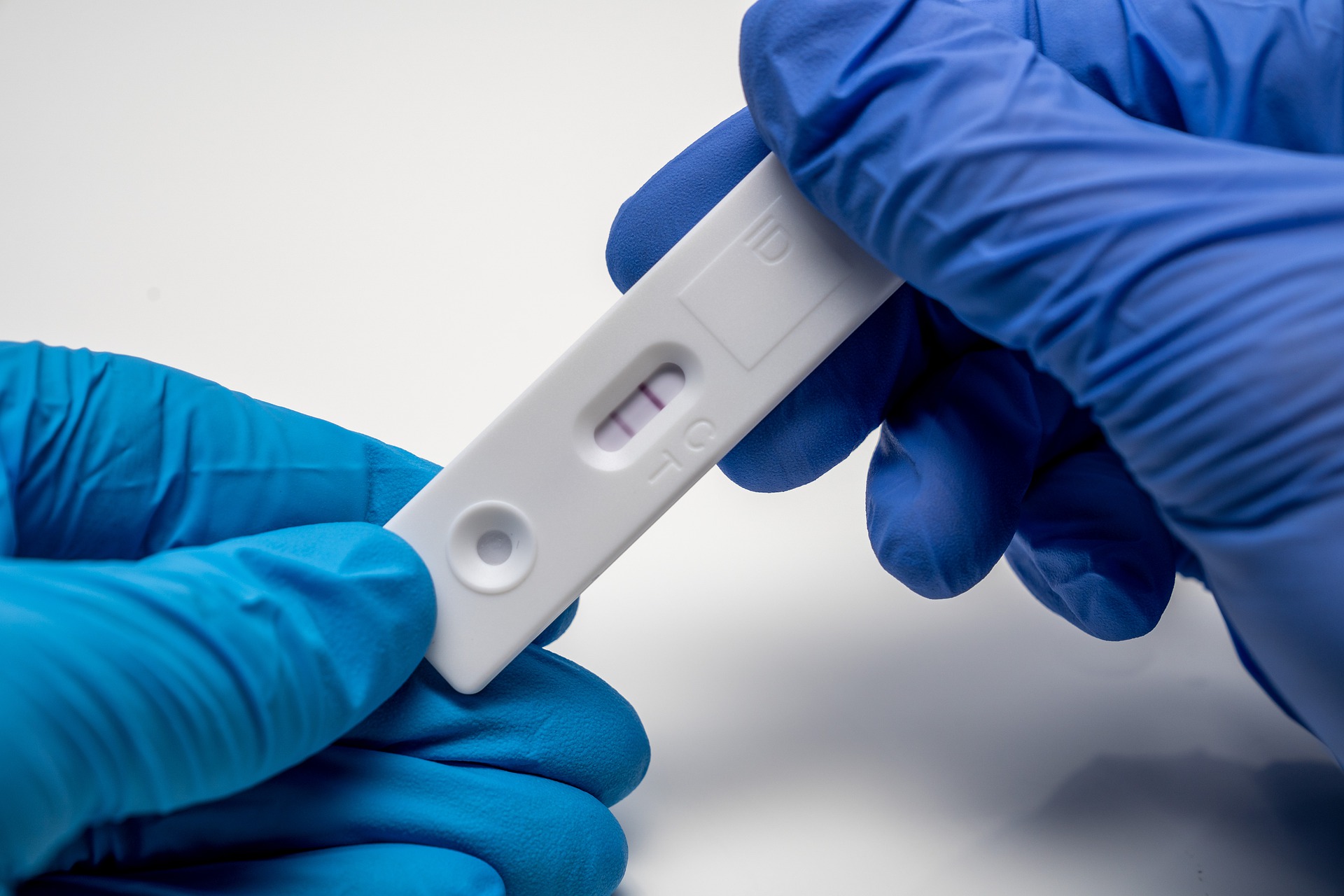
The FDA cautioned in late 2021 after the omicron variant emerged that antigen tests may have reduced sensitivity, meaning they correctly identify fewer people with COVID-19. Data have continued to accumulate that the tests are not as sensitive to the newer variants of the virus, Stenzel said.
Although Covid-19 is not over yet, the market conditions of nucleic acid and antigen detection reagents are rapidly cooling down, and even "cliff-like" subsidence.

The US Food and Drug Administration this week granted Emergency Use Authorization for LG Chem's AdvanSure SARS-CoV-2 IgG(RBD) ELISA COVID-19 antibody test.

On May 19, the COVID-19 Antigen Rapid Test (colloidal gold method) independently developed by Guangzhou Weimi Bio-Tech Co., Ltd (“Weimi Bio-Tech”) successfully obtained the EU CE certificate.

Nanomix said on Friday that it has received the CE mark for its eLab COVID-19 antigen test which provides qualitative results in 15 minutes from an anterior nasal swab.

LumiraDx on Thursday announced it has obtained CE marking for its five-minute SARS-CoV-2 Ag Ultra Test.

The Food and Drug Administration published a Class I recall report for two COVID-19 testing products that covers the removal of more than 51,000 unauthorized coronaviurs tests from the U.S. market.

On May 18, Yunkang Group was listed on Hong Kong Exchanges and Clearing Limited (HKEX), stock code: 2325.HK. At press time, it opened down over 10% with a total market capitalisation of approximately HK$4.3 billion.

Achiko announced Tuesday that it has obtained the CE mark for its COVID-19 AptameX test and system, enabling its use in the European Union and other regions that accept the designation.
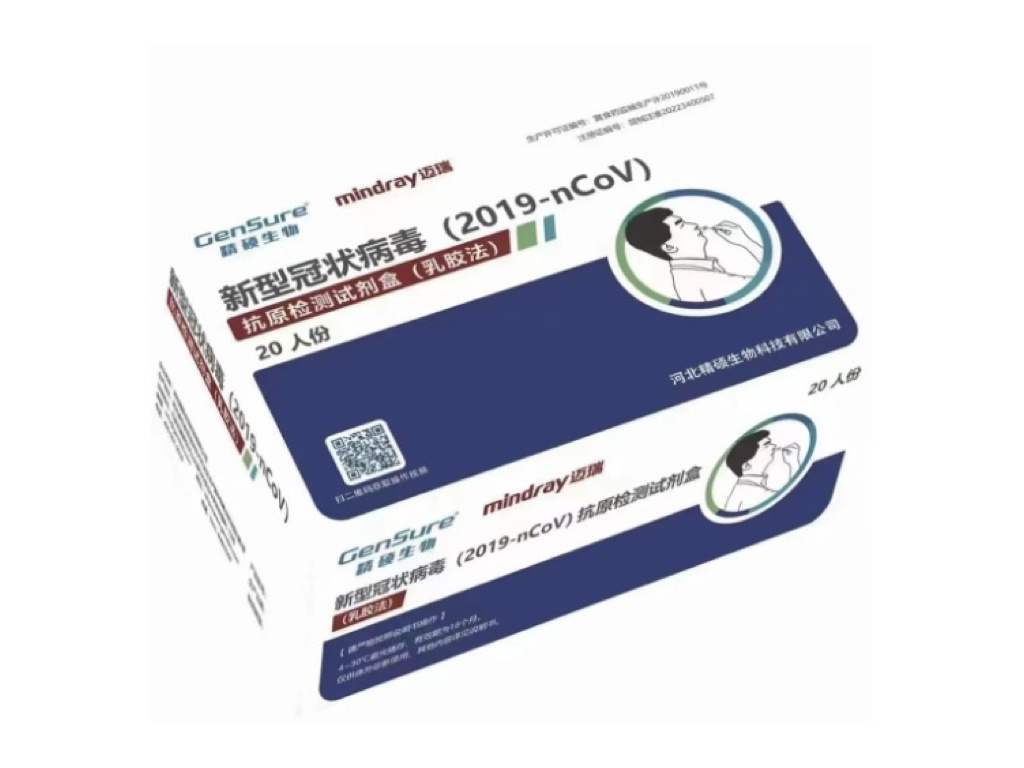
According to industry insiders, Mindray became the general agent of GenSure Biotech Inc., and officially started entered the SARS-CoV-2 antigen test business.
✔ All (65)
✔ Press release (0)
✔ Industry news (65)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
