
The development of modern immunoassay benefited from the development of labeled immunological technology. The clinical problems, which classical immunoassay could not solve, can be solved by modern immunoassay.
In recent years, the market scale of immunodiagnostic reagents in China has been increasing year by year. In 2020, the market size of China's immunodiagnostic reagent industry reached 32.1 billion yuan, an increase of 24.42% year-on-year.

DiaSorin said Tuesday that it has secured CE marking for an immunodiagnostic assay to aid diagnosis of severe conditions including sepsis, septic shock, kidney diseases, and lower respiratory and urinary tract infections, and the firm is launching the assay in Europe.

A spokesperson from Beijing Strong Biotechnologies, Inc. (hereinafter referred to as BSBE) recently responded to investors' questions about chemiluminescence at the 2022 Annual Investor Exchange.
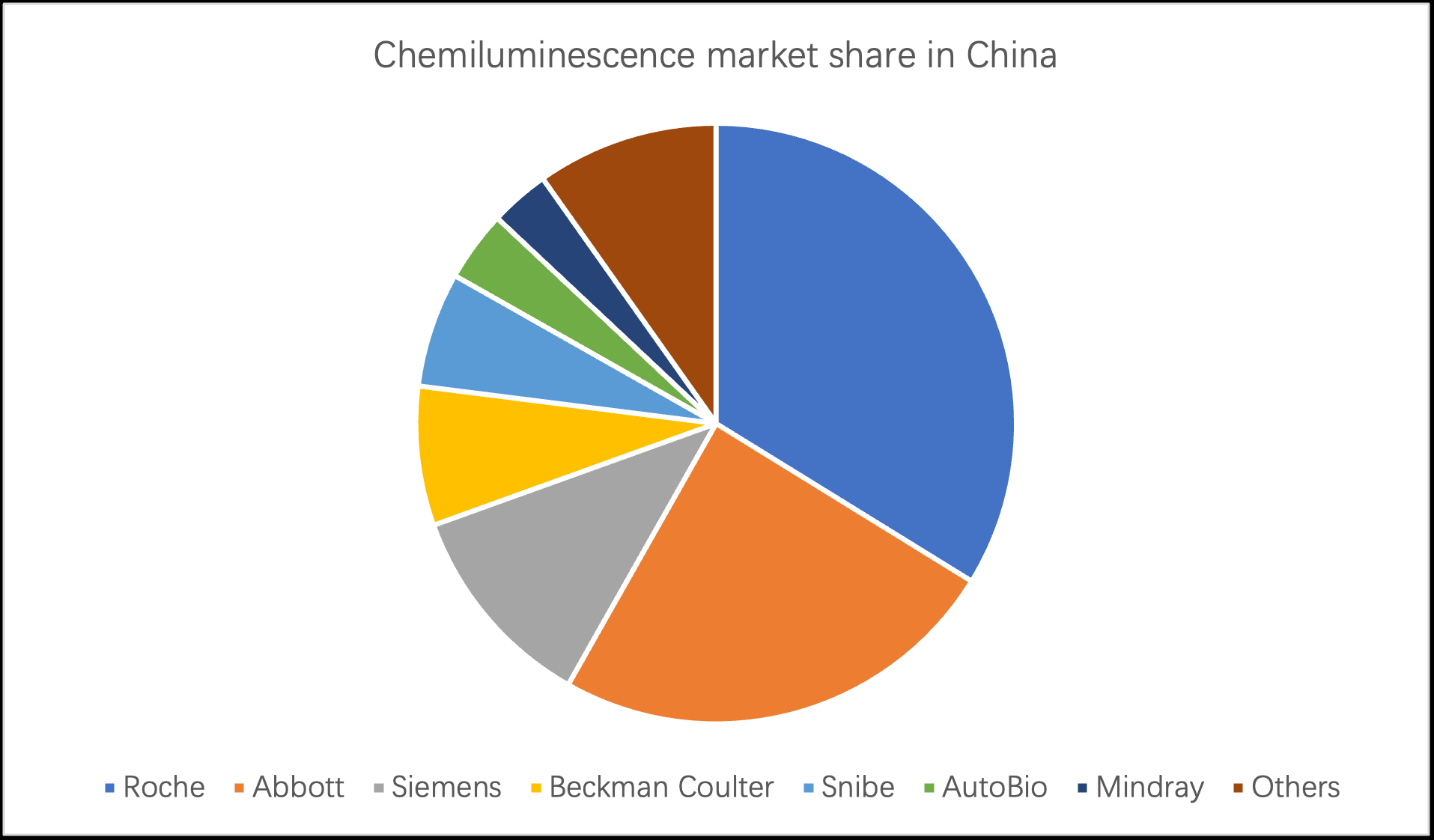
Chinese immunodiagnostics market has maintained an overall growth rate of approximately 20% over the past five years, and is expected to reach a market size of CNY 52.4 billion in 2022.

On March 30, China National Medical Device Co., Ltd. (CNMD for short) under Sinopharm Group and Suzhou Hybiome Biomedical Engineering Co. Ltd., subsidiary of BioMerieux in China held a signing ceremony of the strategic cooperation agreement.

Wondfo Biology Co., Ltd., and Shenzhen Tisenc Medical Device Co., Ltd., a domestic chemiluminescence enterprise, have signed an agreement to acquire 100% equity and corresponding interests of Tisenc medical.

Recently, TÜV SÜD Product Service GmbH an EU Notified Body certified the first IVDR CE certificate in the field of chemiluminescence in China, and its winner is Shenzhen New Industries Biomedical Engineering Co., Ltd.
✔ All (18)
✔ Press release (0)
✔ Industry news (18)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
