
QIAGEN (NYSE: QGEN; Frankfurt Prime Standard: QIA) today announced that its QIAstat-Dx syndromic testing systems and associated assays have received CE-marking under the European Union's new In-Vitro Diagnostic Medical Devices Regulation (IVDR).

Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today the launch of the cobas® Respiratory flex test, the first to use Roche’s novel and proprietary TAGS (Temperature-Activated Generation of Signal) technology.

China's respiratory testing market shows significant growth potential, driven by increasing demand, technological advancements, supportive policies, and a growing market size.

Diasorin (FTSE MIB: DIA) announces that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the company’s NxTAG® Respiratory Pathogen Panel (RPP) v2. This updated panel, an addition to Diasorin’s expanding molecular multiplexing portfolio, responds to customer needs by enhancing test usability.

QIAGEN (NYSE: QGEN; Frankfurt Prime Standard: QIA) today announced that the U.S. Food and Drug Administration (FDA) has cleared the QIAstat-Dx Respiratory Panel Plus syndromic test for clinical use.

DiaSorin has received the US Food and Drug Administration’s 510(k) clearance for its LIAISON PLEX platform and the accompanying LIAISON PLEX Respiratory flex assay.

Thermo Fisher Scientific Inc., the world leader in serving science, today announced the launch of the Thermo Scientific™ KingFisher™ Apex™ Dx, an automated nucleic acid purification instrument, and Applied Biosystems™ MagMAX™ Dx Viral/Pathogen NA Isolation Kit for the isolation and purification of viral and bacterial pathogens from respiratory biological specimens. Together these products provide laboratories with an in vitro diagnostic (IVD) and in vitro diagnostic regulation (IVD-R) approved automated sample preparation solutions for increased confidence in downstream results.
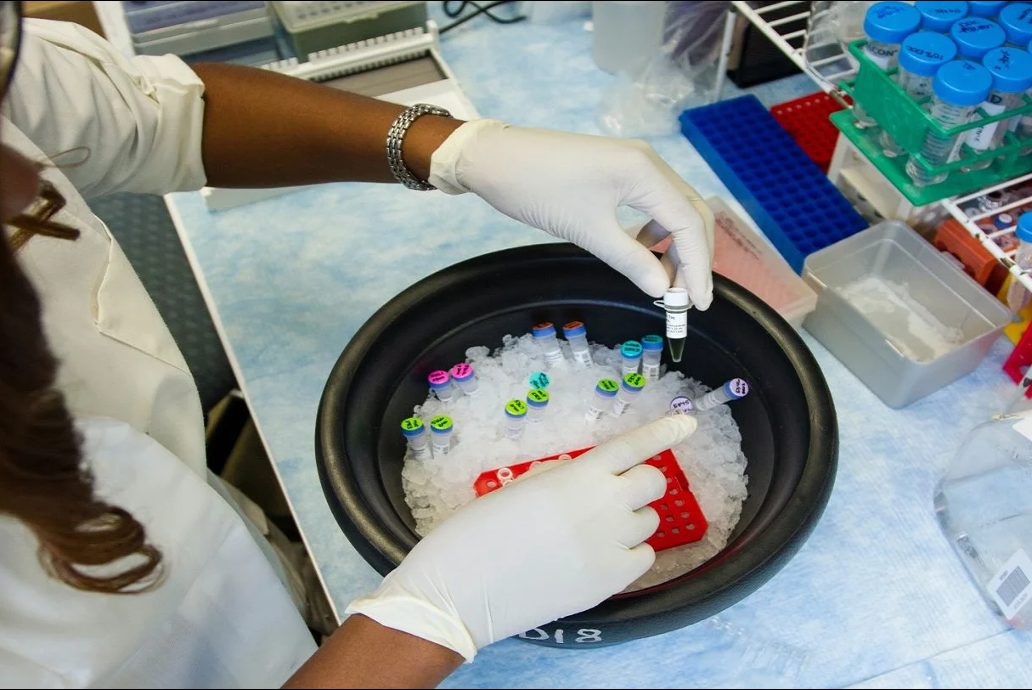
The US Food and Drug Administration (FDA) has granted approval for Lumos Diagnostics’ FebriDx rapid point-of-care (POC) test.

BioMérieux announced on Tuesday that its BioFire SpotFire Respiratory Panel Mini has obtained CLIA wavier from the US Food and Drug Administration.

Qiagen said Monday that its QIAstat-Dx syndromic testing system is expected to be available soon in Japan with a SARS-CoV-2 Respiratory Panel that can detect more than 20 pathogens from a patient sample.
✔ All (24)
✔ Press release (0)
✔ Industry news (24)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
