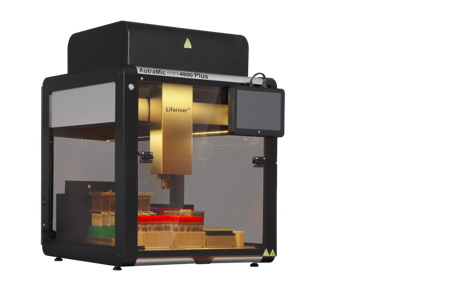
Recently, the AutraMic mini4800 Plus developed by Shanghai ZJ Bio-Tech Co.,Ltd. was approved by National Medical Products Administration. It was the smallest and more automated integrated magnetic bead method + fluorescent PCR nucleic acid detection system.

Chinese sequencing tech company GeneMind Biosciences said earlier this month that its single-molecule DNA sequencer, GenoCare 1600 (originally developed by Direct Genomics), has been approved by China's National Medical Products Administration (NMPA) for clinical and diagnostic applications.

On April 13, National Medical Products Administrations (NMPA) reviewed and approved Zhuhai Livzon Diagnostics Inc. and Shanghai BioGerm Medical Technology Co., Ltd's COVID-19 Antigen Test.

In January 2021, Mindray Medical signed an AI cooperation framework agreement with Tencent AI Lab.
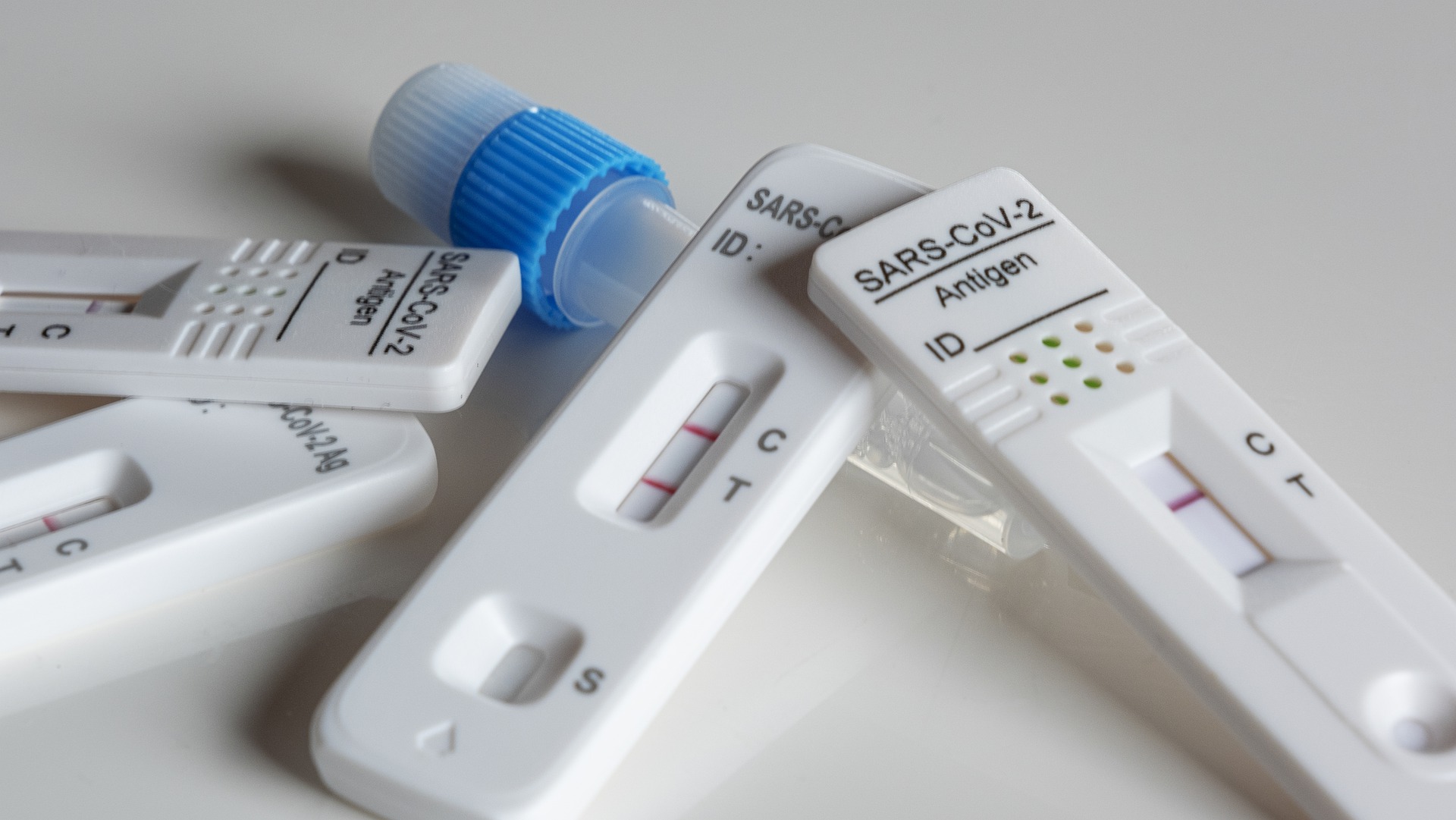
On March 17th, NMPA has approved the 14th New Coronavirus (2019-nCoV) antigen detection kit (colloidal gold method) produced by Zybio. Up to now, 14 COVID-19 antigen detection kits have been approved for sale.

Jiangsu Mole Bioscience obtained the approval of the Helicobacter pylori 23S rRNA gene and gyrA gene mutation test kit (fluorescence PCR method) by NMPA. This kit is the first product in China that can simultaneously detect clarithromycin and levofloxacin resistance genes.

AnPac Bio-Medical Science, a cancer screening and detection company, said on Monday that it has filed for registration testing of its Class III, multi-cancer detection device with China's National Medical Products Administration.

China's National Medical Products Administration (NMPA) recently extended approval of the Roche Cobas EGFR Mutation Test v2 to include the use of plasma samples for the detection of EGFR mutations in patients with non-small cell lung cancer,
✔ All (38)
✔ Press release (0)
✔ Industry news (38)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
