
Burning Rock Biotech Limited (NASDAQ: BNR and LSE: BNR, the “Company” or “Burning Rock”) is pleased to announce that followed an earlier Breakthrough Device Designation granted by the US Food and Drug Administration (FDA) for its OverC™ Multi-Cancer Detection Blood Test (MCDBT) in January 2023, its OverC™ MCDBT has been granted Breakthrough Device Designation by the China National Medical Products Administration(NMPA), which represents the only test globally that has received Breakthrough Device Designation from both US FDA and China NMPA.

As the demand for the mass spectrometry market in China has rapidly increased, major domestic and international mass spectrometer manufacturers have accelerated their product registration efforts. In recent years, a variety of different types of mass spectrometry instruments have obtained NMPA certificates.

MGI Tech said last week that its DNBSeq-G99 sequencer has been approved as a medical device by China's National Medical Products Administration (NMPA).
On 5 July 2023, National Medical Products Administration announced the imported Class I medical device product in June 2023. A total of 145 product information, including 7 products in the IVD industry, were finally released.

On July 10, 2023, NMPA released information on the delivery of medical device approval certification documents.

Helio Genomics said Thursday that its sister company, Laboratory for Advanced Medicine & Health Group (LAMH), has received approval from the National Medical Products Administration (NMPA) in China for its cell-free DNA-based liver cancer detection test. The assay is the first of its kind to receive this approval.

Recently, U.S. Food and Drug Administration (FDA) and China National Medical Products Administration (NMPA) have released their summaries on medical device innovation in 2022.

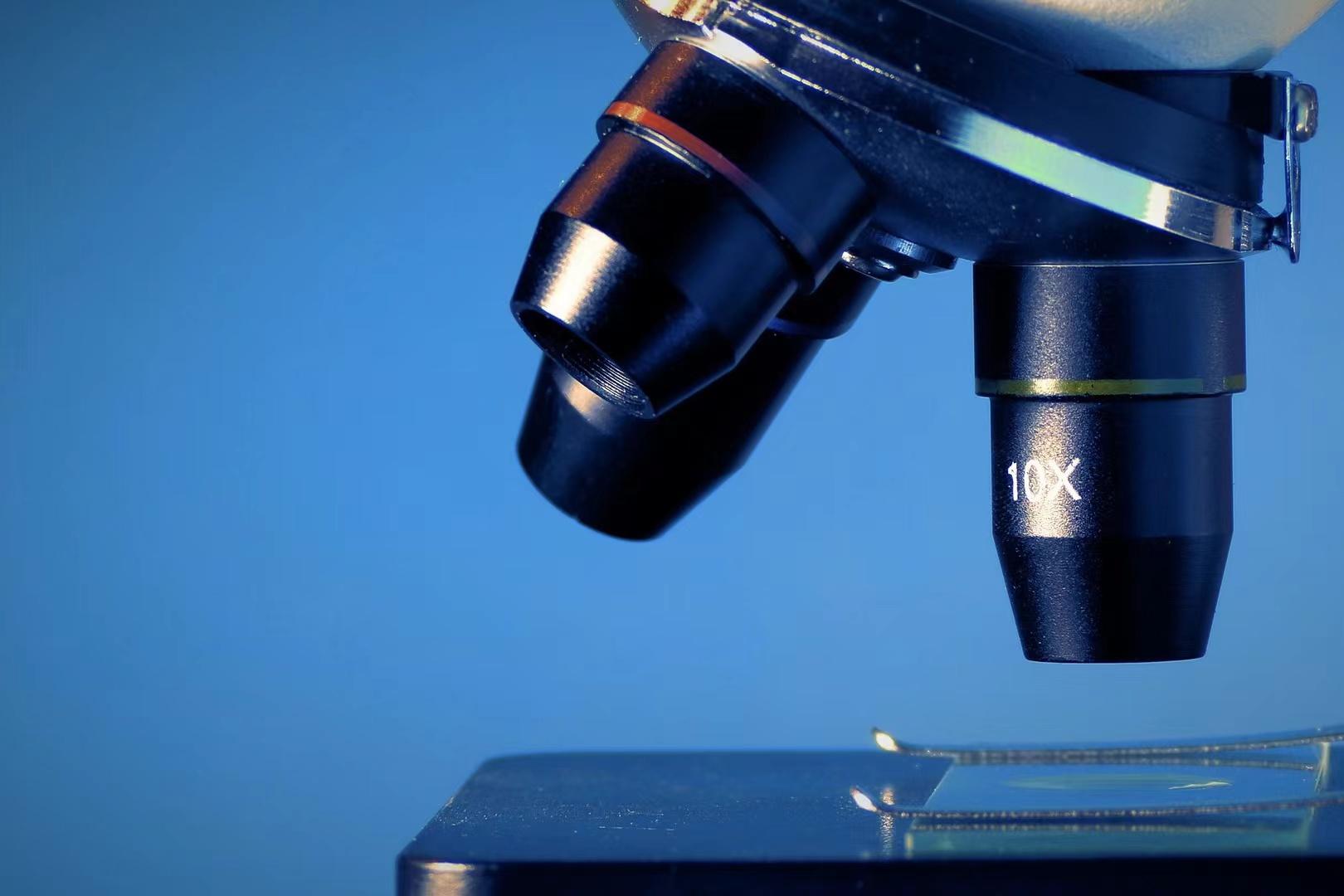
On February 7 2023, RocGene's independently developed Archimed Mini 16 Medical Fluorescence Quantitative PCR Instrument obtained the Class III Medical Device Registration Certificate by the National Medical Products Administration.

On January 5, 2023, Guangzhou Darui Biotechnology Co., Ltd., the subsidiary of Daan Gene Co., Ltd., received China National Medical Products Administration (NMPA) class III medical device approval for its “Twenty Genetic Deafness Genes Mutation Detection Kit (Time of Flight Mass Spectrometry)”.

On Dec. 1st, Sansure announced that its bordetella pertussis nucleic acid test kit (PCR-fluorescent probe method) obtained the certificate approved by NMPA.
✔ All (38)
✔ Press release (0)
✔ Industry news (38)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
