


China will adjust tariffs on imported U.S. products from 12:01 p.m. Wednesday, the Customs Tariff Commission of the State Council announced on Tuesday.

The immunoassay segment accounts about 35% of the market, with no growth compared to the previous year. Domestic brands account for over 35%.
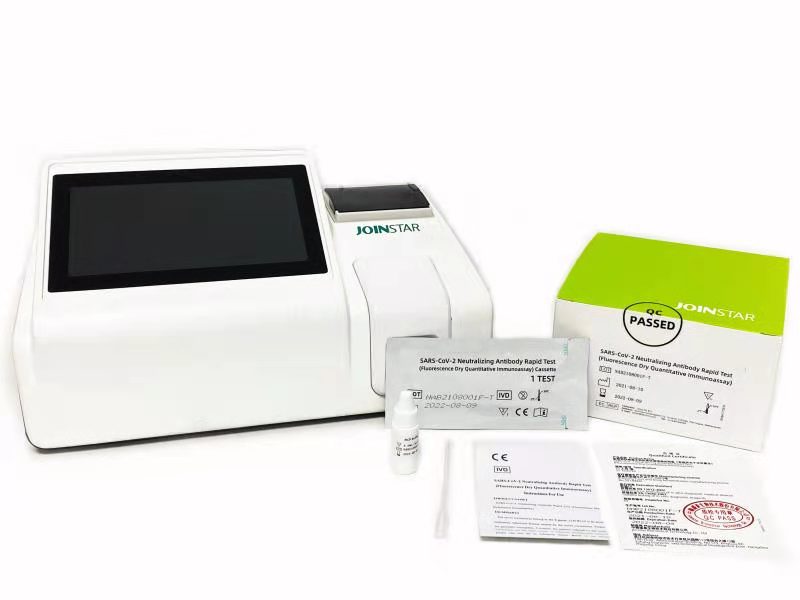
Recently, SARS-CoV-2 Neutralizing Antibody Rapid Test (Fluorescence Dry Quantitative Immunoassay) developed by Joinstar Biomedical Technology Co., Ltd. and Nanshan Pharmaceutical Research Institute of Guangdong province was officially sold to overseas markets.

On August 21, TELLGEN posted a 157.93% year-over-year increase on continued demand for its tumor and autoimmunity test.

AnPac Bio-Medical Science, a cancer screening and detection company, said on Monday that it has filed for registration testing of its Class III, multi-cancer detection device with China's National Medical Products Administration.

Point-of-care diagnostics firm LumiraDx and special purpose acquisition company CA Healthcare Acquisition said on Monday that they have revised the terms of their previously announced merger to lower the equity value of the combined company.
Waltham, MA-based Thermo Fisher Scientific was granted emergency use authorization for the TaqPath COVID-19 Fast PCR Combo Kit 2.0 and the TaqPath COVID-19 RNase P Combo Kit 2.0 assays.

The European Commission has opened an investigation assess whether Illumina's decision to complete its acquisition of Grail is a breach of the “standstill obligation”

Amoy Diagnostics and Amgen on Thursday said that they have entered a strategic partnership to develop a companion diagnostic for the Japanese market to identify non-small cell lung cancer patients for Amgen's KRAS G12C inhibitor, sotorasib (Lumakras).
Partek said that it has entered into an agreement with Santa Clara, California-based Agilent Technologies to integrate its Partek Flow bioinformatics software with the Agilent Alissa Clinical Informatics platform, providing an end-to-end analysis workflow that is customizable for Agilent's customers

As the first share of POCT in China, Wondfo will continue to be driven by innovation and cooperation in 2021, implement both epidemic business, conventional business, and double-cycle in domestic and abroad.

South Korean diagnostics company Seegene on Wednesday reported a nearly 11 percent year-over-year increase in its second quarter revenues amid higher sales of its infectious disease testing reagents and PCR equipment in Europe and North America.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
