Although both share a preference for use of oral solid-dosage forms, the business model for consumer pharmaceutical manufacturers—often called over-the-counter (OTC) manufacturers in the United States—differs significantly from that of prescription drug manufacturers. However, both types of companies have been responding to market changes by focusing on formulation and delivery changes that increase convenience to the patient/consumer. Recent trends in consumer-directed innovation in the OTC pharmaceutical market are summarized in Figure 1.
Differences between prescription and OTC products first became pronounced in the mid-1980s, when OTC manufacturers began to introduce increasingly diverse consumer-oriented products, and to change their approach to marketing, promotion, and packaging. The OTC marketing approach became more like that used in other fast-moving consumer packaged goods (FMCG) industries (e.g., foods, beverages, and personal care products). Packaging and advertising were increasingly directed to the consumer, rather than the healthcare provider or pharmacist.
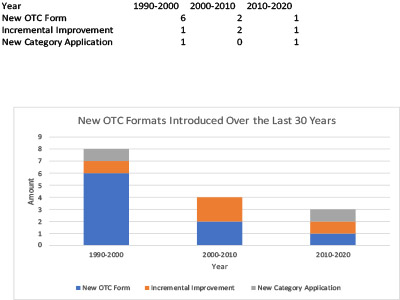
Figure 1. New OTC Formats, 1990-2020 (Figure courtesy of the author).
Emphasis on form and sensory attributes
During the 1990s, the marketing of dosage formats and features such as form and sensory attributes (e.g.,colors, appearance, and flavors) became part of the product promotion mix. Recently, a major growth driver for OTC pharmaceuticals, particularly in the US, has been new products that resulted from prescription-to-OTC switches. These products were predominantly standard tablets or capsules with relatively few new delivery formats other than some modified-release products, the most unique being a slow-release nicotine chewing gum.
Introduction of more competitive and higher quality private-label or store-brand products in the 1990s underscored the need for branded OTC manufacturers to differentiate their offerings. The pressure to innovate with dosage form and packaging design has remained constant, driven by competing OTC brands as well as generic-pharmaceutical companies that supply drug stores and supermarkets with copies of branded products. Retailer consolidation has also driven increased competition, and dosage form innovation has become a crucial way for companies to differentiate products (1).
Greater consumer focus in OTC products
Both product packaging and delivery form have become key differentiators for OTC products. Traditional syrup, tablet, and capsule formats have made way for a diverse array of forms such as softgels, gelcaps, chewing gums, powders, effervescent tablets for hot and cold drinks, as well as orally disintegrating tablets and confectionery-derived forms.
A number of considerations go into the consumer’s buying decision (2). Delivery format may often reflect patient compliance issues, especially for pediatric formulas, but perceptions of efficacy and consumer preference are also crucial. More complex OTC formulations and delivery systems often require unique manufacturing technology and almost always involve complex analytical methodologies due to the additional excipients used in the formulations.
Most orally administered products that switched from prescription to OTC did not introduce new delivery formats. However, many brought significant consumer benefits (e.g., less frequent dosing) to existing categories such as longer-acting analgesics. An example would be Bayer’s longer lasting Aleve.
They also created new paradigms for consumers (e.g., once daily dosing for heartburn and seasonal allergy treatments). These product introductions greatly increased the consumer’s awareness and understanding of the benefits of longer-acting remedies and less-frequent dosing. As a result, consumers expect more from OTC treatments. Continuous innovation in packaging and drug delivery formats is required if a new OTC franchise is to remain successful (3).
More convenient dosing
Around 30 years ago, several sustained-release cough cold brands that had dosing of 12 hours, were developed and marketed, such as Drixoral (originally Schering-Plough’s trade name), and Delsym (Fison’s brand in the 1980s, but now Reckitt Benckiser’s [RB’s]). These products may have been ahead of their time because most OTC products in the mid-1980s were dosed at a 4–6-hour frequency. It was not until the 1990s, after several longer-acting drugs switched from prescription to OTC, that consumers became familiar with once- or twice-daily dosing. Thus, Delsym and other legacy long-acting brands experienced something of a renaissance in the late 1990s and early 2000s.
Johnson & Johnson’s Tylenol analgesic brand maintained a strong market presence over several decades, due to continuous form innovation, which included introducing novel gelatin-coated, capsule-shaped tablets called gelcaps, with a later addition of gelatin-coated round tablets called geltabs. In the mid 2000s, Tylenol gelcaps were replaced by rapid-release gels, gelatin-coated caplets with laser-drilled holes that enabled faster disintegration and dissolution. Other innovations included improving the taste of pediatric analgesics by replacing liquid formulations with suspensions, thus improving patient compliance.
For solid-dose formulations, taste-masked drug particles were incorporated into chewable tablets, which offered a convenient dosage form for both children and adults. Taste-masked drug particles also enabled the convenient powder pack dosage format introduced in 2019 as Children’s Tylenol dissolve packs. Another innovation in the cough/cold category was the hot-drink format first introduced in the UK under the Lemsip brand(now owned by RB) and later in the US as Theraflu (Sandoz’s brand in the 1990s but GlaxoSmithKline’s today).

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
