


Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

China will adjust tariffs on imported U.S. products from 12:01 p.m. Wednesday, the Customs Tariff Commission of the State Council announced on Tuesday.

MGI Tech said last week that its DNBSeq-G99 sequencer has been approved as a medical device by China's National Medical Products Administration (NMPA).

Ibex Medical Analytics (Ibex) has launched a breast cancer biomarker scoring tool powered by artificial intelligence (AI) to help clinicians with treatment decisions.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

Paige, a technology disruptor in healthcare, has joined forces with Microsoft in the fight against cancer, making headway in their collaboration to transform cancer diagnosis and patient care by building the world’s largest image-based artificial intelligence (AI) models for digital pathology and oncology.

Novartis subsidiary Navigate Biopharma announced on Friday that it has formed a strategic collaboration with Becton Dickinson to help develop and commercialize companion diagnostics and clinical decision-making tools using flow cytometry.

As a global company with a broad range of businesses, operations in over 160 countries and 115,000 people around the world, diversity is inherent to our business and organization.

Laborie Medical Technologies has entered a definitive agreement for the acquisition of medical device company Urotronic in a deal valued at up to $600m.
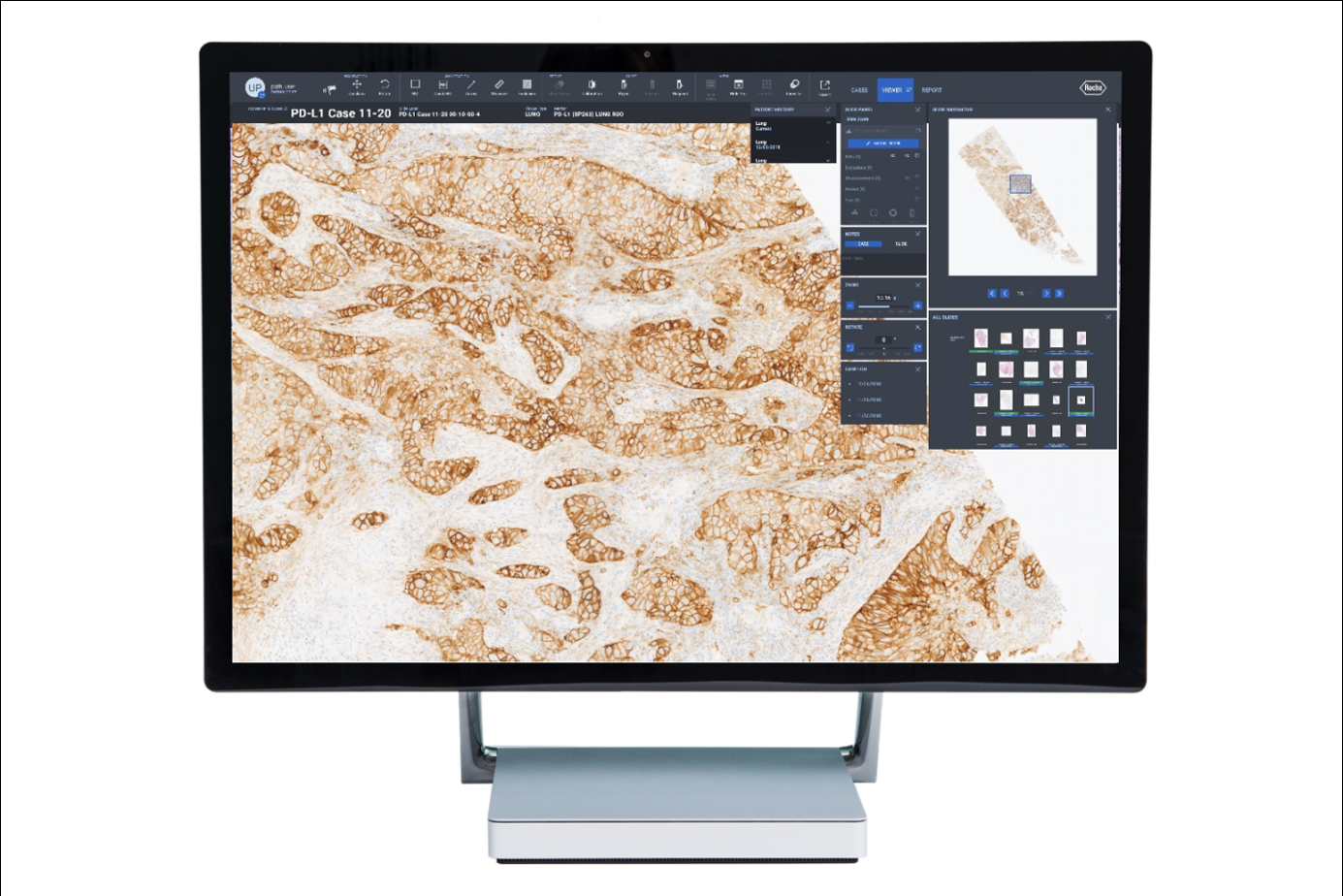
On September 6th, Roche Diagnostics China announced its official partnership with Deep Informatics. They will jointly launch a pathology artificial intelligence (AI) assisted interpretation algorithm product, further promoting the digitalization and intelligent transformation of pathology diagnosis in China, providing strong support for the development of personalized precision diagnosis and treatment.

Circular Genomics, the global leader in circular RNA biomarkers for precision psychiatry, has announced a partnership with leading Alzheimer’s genomics expert Dr. Carlos Cruchaga to explore the role of circRNAs as blood biomarkers for early diagnosis and treatment of Alzheimer’s Disease.

US Food and Drug Administration officials on Wednesday released draft guidance outlining the types of predicate device analysis and evidence they will expect in 510(k) clearance applications as they work to modernize the clearance process for medical devices, including in vitro diagnostics.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
