


China will adjust tariffs on imported U.S. products from 12:01 p.m. Wednesday, the Customs Tariff Commission of the State Council announced on Tuesday.

The immunoassay segment accounts about 35% of the market, with no growth compared to the previous year. Domestic brands account for over 35%.
China Association of In Vitro Diagnostics (CAIVD) and In Vitro Diagnostics Society of China Association for Medical Devices Industry jointly compiled this report.

Despite concerns from researchers and organizations, the U.S. National Institutes of Health (NIH) is adamant about implementing its new mandate requiring foreign partners to share raw data and notebooks at regular set intervals with their primary U.S. grant recipient, releasing its final guidance on September 15.

Streck announced on Monday that its MDx-Chex quality controls have received clearance from the US Food and Drug Administration.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.
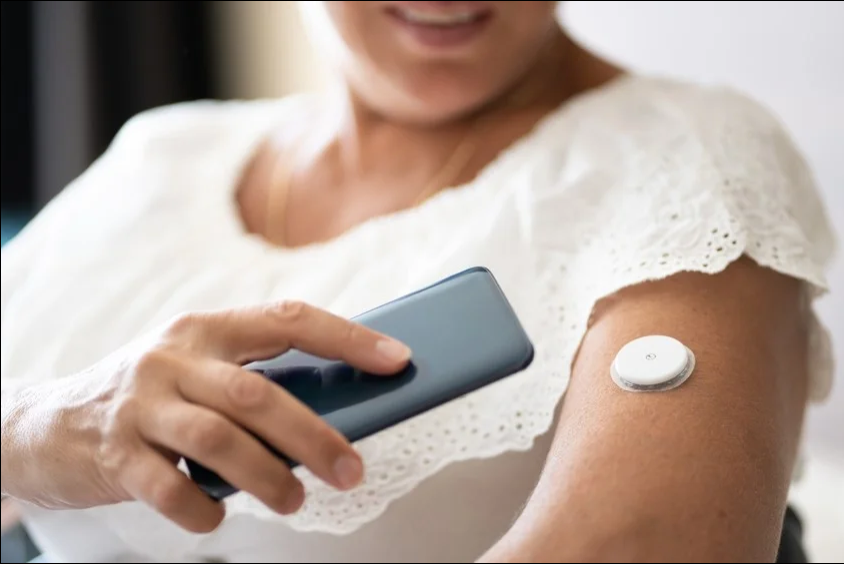
Medtronic has received a CE mark for its continuous glucose monitoring (CGM) device, Simplera, allowing the device to be marketed in the European Union.

Abbott (NYSE: ABT) today announced it has completed the acquisition of Bigfoot Biomedical, a leader in developing smart insulin management systems for people with diabetes.

As the demand for the mass spectrometry market in China has rapidly increased, major domestic and international mass spectrometer manufacturers have accelerated their product registration efforts. In recent years, a variety of different types of mass spectrometry instruments have obtained NMPA certificates.

A systematic review of earlier studies has found that artificial intelligence (AI) and machine learning (ML) can support the diagnosis of polycystic ovarian syndrome (PCOS).

QuidelOrtho Corporation (Nasdaq: QDEL) (“QuidelOrtho”), a global provider of innovative in vitro diagnostic technologies designed for point-of-care settings, clinical labs and transfusion medicine, announced today that it has been granted CLIA Waiver from the U.S. Food and Drug Administration (FDA), applying to its new Sofia® 2 SARS Antigen+ FIA.

Myriad Genetics, Inc. (NASDAQ: MYGN), a leader in genetic testing and precision medicine, today announced two key milestones in its strategic partnership with Illumina Inc. (NASDAQ: ILMN), a global leader in DNA sequencing and array-based technologies, to advance and support clinical research for gene-based, targeted therapies.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
