


Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

China will adjust tariffs on imported U.S. products from 12:01 p.m. Wednesday, the Customs Tariff Commission of the State Council announced on Tuesday.

Oxford Nanopore Technologies PLC (LSE:ONT) has secured a £70 million investment from French in-vitro diagnostics (IVD) company bioMérieux, in exchange for a 3.5% stake.
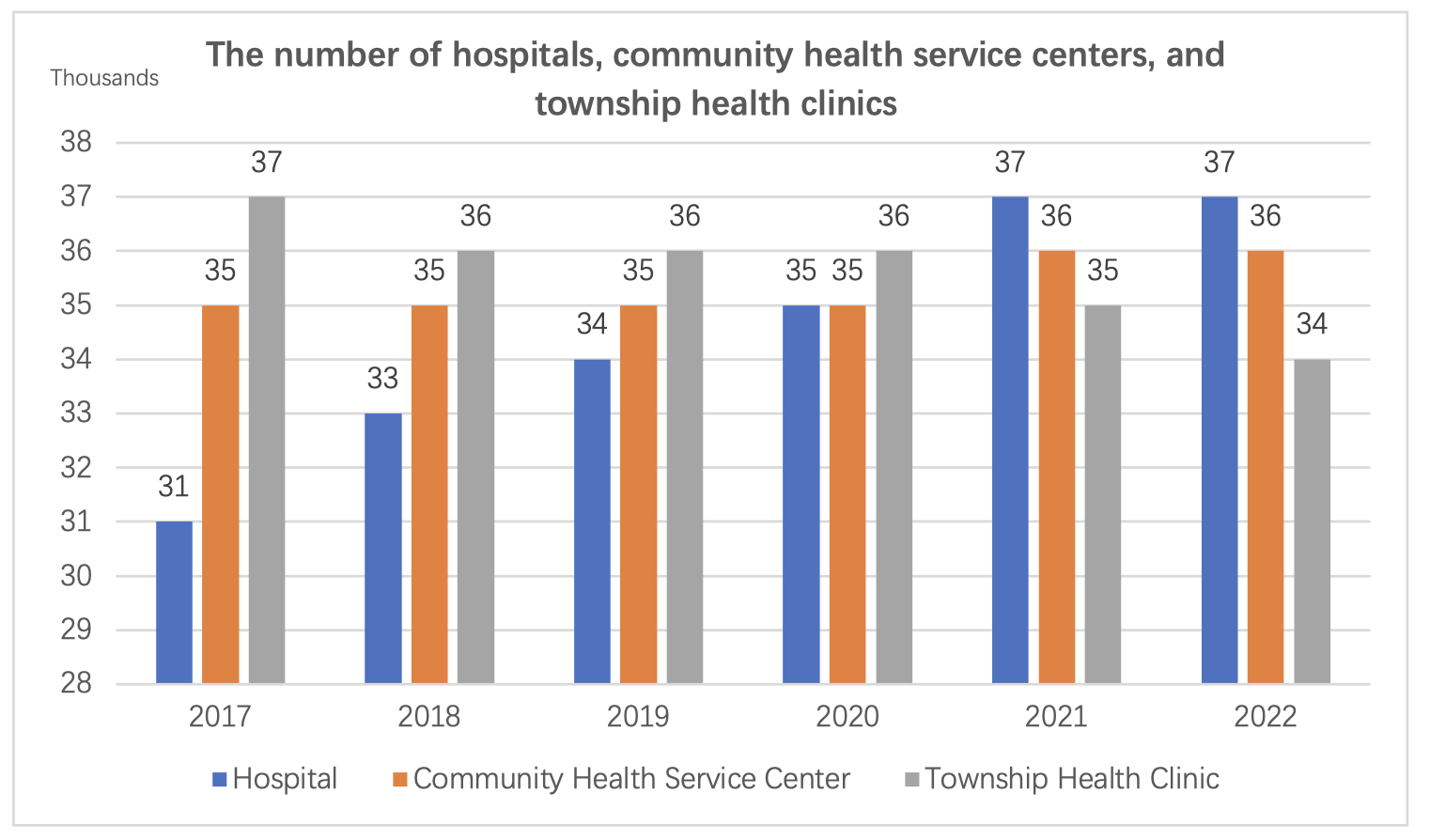
By the end of 2022, the total number of medical and health institutions nationwide reached 1,032,918, an increase of 1,983 compared to the previous year.

Revvity and Danaher subsidiary Sciex said Tuesday that they have signed a distribution agreement that will improve the accuracy of mass spectrometry-based disease screening in newborns by combining Sciex's mass spectrometry instruments and Revvity's reagents.

Abbott (NYSE: ABT) today announced financial results for the third quarter ended Sept. 30, 2023.

As of October 12, 2023, a total of 231 breakthrough medical devices have been approved in China, including 24 IVD products.
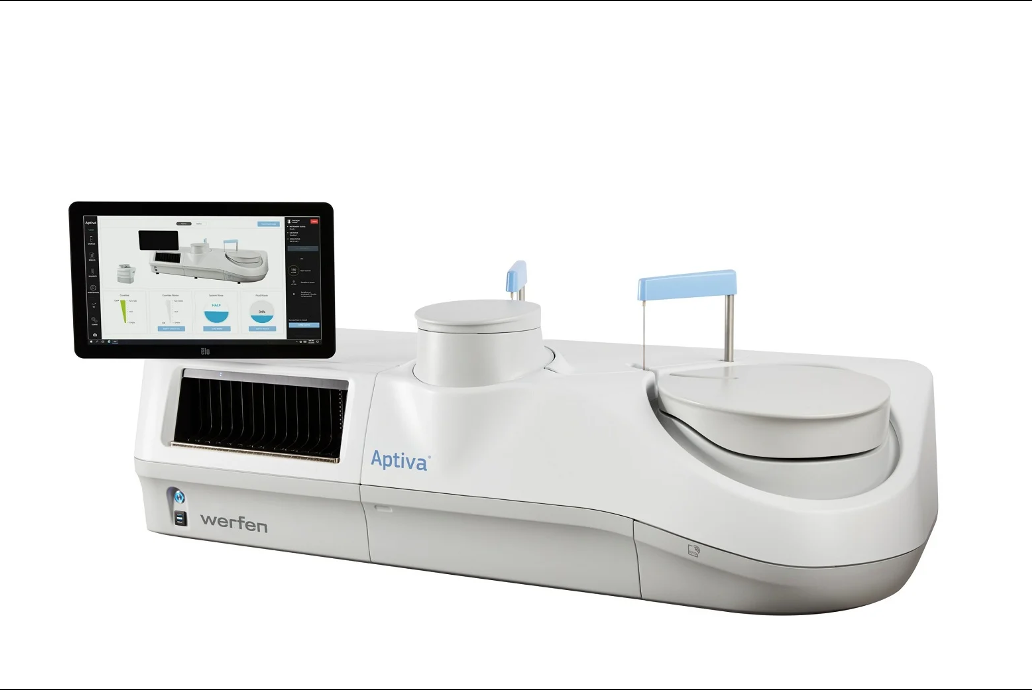
Werfen has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Aptiva Connective Tissue Disease (CTD) Essential reagent.

Thermo Fisher Scientific Inc. (NYSE: TMO) (“Thermo Fisher”), the world leader in serving science, and Olink Holding AB (publ) (“Olink”) (Nasdaq: OLK), a leading provider of next-generation proteomics solutions, today announced that their respective boards of directors have approved Thermo Fisher’s proposal to acquire Olink for $26.00 per common share in cash, representing $26.00 per American Depositary Share (ADS) in cash.
China Association of In Vitro Diagnostics (CAIVD) and In Vitro Diagnostics Society of China Association for Medical Devices Industry jointly compiled this report.

EliTechGroup announced on Thursday that it has received certification under Europe's In Vitro Diagnostic Regulations for multiple infectious disease tests.

Revvity, Inc. and Element Biosciences, Inc., a developer of the AVITI™ System, an innovative and emerging genomic sequencing platform, today announced a collaboration to introduce workflow solutions that save time and effort required for genomic analysis of samples.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
